A blood test for fasting insulin can be one of the most important that you get.
It’s important because it shows the degree of insulin resistance: the higher the number, the more insulin resistant. A very high number generally means type 2 diabetes. A number merely higher than normal can signify metabolic syndrome or pre-diabetes.
Hyperinsulinemia, or too much insulin in the blood, is one of the main causes of chronic disease in the modern world, including heart disease, cancer, kidney disease, and of course diabetes.
Being sedentary leads to insulin resistance, and exercise can prevent it.
Insulin resistance is strongly associated with obesity, but normal weight people, especially those who are skinny-fat, can have it too.
A diet high in sugar and refined carbohydrates, especially when combined with seed oils, leads to hyperinsulinemia and insulin resistance.
My fasting insulin test
Doctors don’t routinely test for fasting insulin; they normally do so only if they suspect diabetes.
I’ve had a few tests of fasting blood glucose that were high, in the range of 100 to 110. That’s an odd result, because I eat a low-carbohydrate diet and lift weights, and have a body fat percentage that I don’t know exactly but is likely <15%.
It’s probably due to the so-called dawn phenomenon, or physiological insulin resistance, which is normal. When on a low-carbohydrate diet, the liver can become insulin resistant in order to make glucose for the rest of the body. To be honest, the causes of the dawn phenomenon are not fully elucidated, and experts give varying explanations. But the fact is that many people who eat low-carb report it.
Another reason for a high fasting glucose can be stress and cortisol; if you go for a blood draw on a morning when you’re rushing off to work, for instance, that could elevate your glucose.
Not being clear on whether I should be concerned about my high fasting glucose, I decided to get a fasting insulin test from Life Extension.
Result: 2.9 µIU/ml. Normal range is 2.6 to 24.9. (On this scale, 1 µIU/ml = 6.9 pmol/L.) Ideal is <3. The odds ratio for prediabetes rises sharply with increased fasting insulin.
My result was close to ideal. I think I’m going to live another few years.
Should I remain concerned about my fasting glucose test? Probably not; my non-fasting glucose is actually lower than my fasting glucose, which would seem to indicate, together with my insulin test, that I have no risk of diabetes. It would indeed be strange if I did have increased risk, for the reasons mentioned above: low-carb diet, weight lifting, low body fat, plenty of muscle too.
If you do have a high fasting insulin, then you need to get to work. Below are relative risks of hypertension, high triglycerides, and diabetes based on fasting insulin levels. (Source.)
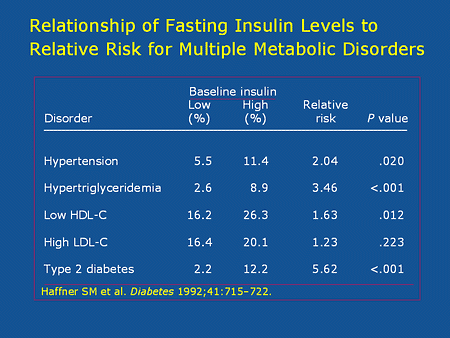
To lower a fasting insulin value, two interventions are important:
- Avoid sugar and refined carbohydrates
- Exercise.
You can order a fasting insulin test through Life Extension; blood is drawn at no extra charge at LabCorp.













26 Comments
I had the same thing in my most recent blood test, good cholesterol figures, rock-bottom triglycerides (45.6 mg/dL), but a blood-glucose reading of 99 mg/dL, which is borderline high. I’m not skinny-fat (waist/height ratio of 0.45, low body fat), so it was a bit concerning.
My doctor told me not to worry about it, and the Dawn Phenomenon makes some sense, but I’ll ask for an insulin test next time I get my bloodwork done. Still, I’m thinking that the combination of optimal cholesterol, low TG, and low body fat indicates there’s nothing amiss.
Worth checking your Glucagon levels.
Rare pancreatic and GI tumours releasing glucagon can cause these abberrant high Blood sugars.
PD have you looked into IGF1? In my understanding it has a similar role to insulin, and controls fasting glucose. E.g. when pituitary gland releases HGH during sleep, it raises blood glucose, and IGF1 is released to drive it into cells.
I read that certain B vitamins are needed for liver to produce IGF1 which will control blood glucose between meals. I will post some links below with summaries.
https://jdmdonline.biomedcentral.com/articles/10.1186/2251-6581-12-17 – Vitamin B12 deficiency common among T1D and T2D patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1264530 – page 10: in rats optimum vitamin B1 requirement to ensure normal carbohydrate metabolism is many times the ordinary protective dose.
https://care.diabetesjournals.org/content/28/1/120 – Plasma Concentration of IGF-I Is Independently Associated With Insulin Sensitivity in Subjects With Different Degrees of Glucose Tolerance. IGF-I has the characteristics to be a marker for the insulin resistance syndrome. This suggests that low IGF-I levels may be a useful marker for identifying subjects at risk for cardiovascular disease.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4702316/ – Multiple studies (in vitro and in vivo) demonstrate the association between IGF-1 deficit and deregulated lipid metabolism, cardiovascular disease, diabetes, and an altered metabolic profile of diabetic patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC443374/ – IGF-I administration increased total IGF-I serum levels 5.3-fold above control. During the control period mean fasting glucose, insulin, C-peptide, and total triglyceride levels were 11.0 mmol/l, 108 pmol/l, 793 pmol/l, and 3.1 mmol/l, respectively; and decreased during IGF-1 treatment to 6.6 mmol/l, 47 pmol/l, 311 pmol/l, and 1.6 mmol/l, respectively. Postprandial areas under the glucose, insulin, and C-peptide curve decreased to 77, 52, and 60% of control, respectively.
https://www.idf.org/sites/default/files/attachments/article_61_en.pdf – diabetics have 2-3x higher growth hormone secretion. In T1D insulin does not reach liver to stimulate IGF1 production, which may explain why GH secretion is not controlled (as IGF1 is important in controlling pituitary GH secretion).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4071367/ – Vitamin B12 deficiency decreases Taurine production. Taurine induces GH-dependent IGF1 synthesis in the liver. Therefore, low B12 leads to low Taurine and low IGF-1 and retarded growth.
https://www.ncbi.nlm.nih.gov/pubmed/842585 – Vitamin B6 treatment of gestational diabetes mellitus (pregnancy diabetes); statistically significant improvement in the glucose tolerance curve after the vitamin B6 treatment, with a lowering of blood glucose levels, despite an unchanged or lowered plasma insulin level. Thus a relative deficiency in vitamin B6 is associated with some cases of gestational diabetes mellitus and that the replacement of B6 improves the metabolic state.
https://www.youtube.com/watch?v=VYoj46ifKHw – Dr Darren Schmidt lactic acid cycle. Insulin like growth factor IGF-1 more important than insulin for blood sugar, IGF-1 controls blood sugar between meals, insulin controls blood sugar right after meals. Insulin comes from pancreas, IGF-1 comes from liver. Liver converts lactic acid back to sugar (75%) while rest of lactic acid stays in blood and dilutes arteries and capilaries. Need to maintain 24 B Vitamins (e.g. nutritional yeast or rice bran for full Vitamin B-complex) levels for healthy liver and detox it from metals etc, which will likely affect IGF-1 production.
From comments:
“Diabetics typically have elevated lactate, which shows that glucose doesn’t have a problem getting into their cells, just getting oxidized.” -Ray Peat, PhD. “Diabetics are relatively unable to oxidize glucose, they produce lactate in the presence of O2, and may synthesize fat inappropriately. Diabetes is relevant to cancer exactly because of their shared inability to oxidize sugar and lactic acid.” -Ray Peat, PhD. “The presence of lactic acid in our tissues is very meaningful, but it is normally treated as only an indicator, rather than as a cause, of biological problems. Its presence in rosacea, arthritis, heart disease, diabetes, neurological diseases and cancer has been recognized, and recently it is being recognized that suppressing it can be curative, after fifty years of denial. Lactate contributes to diabetes, inhibiting the ability to oxidize glucose.
Obviously, much has been made of insulin resistance and higher numbers of fasting insulin, but what of “low” numbers? I haven’t been able to ascertain the possible implications, if any, of a lower number than 2.6. I had mine tested 9 months ago and it came up 1.6. Since then, I’ve been at 2.9 as well but I am curious about the 2.6 cutoff point.
Very low insulin numbers could be indicative of type 1 diabetes, but you obviously don’t have that since you’re now above the cutoff.
Yes. I am not so much curious about the 1.6 reading, but I am curious as to why 2.6 is the base metric for “normal.”
Well, it’s probably because of how they compute any lab normal value: 95% of all putatively “healthy” people have a value within that range. Given the state of American health, if you have a value below the normal lower limit, it’s unlikely to be a cause for concern.
Thanx for that insight.
IGF-1 is a helpful thing to know since both high and low numbers are associated with increased mortality, heart disease and cancer.. The typical western diet usually yields an IGF of 200-210. You want it to be under 175, and ideally under 150, but higher than 85 because it’s a U-shaped curve of risk.
My fbs’s are now routinely elevated between 115-120 since I began rapamycin. Both CR and rapamycin are perceived by the body as a starvation state and induce a preferential liver insulin resistance in order to maintain gluconeogenesis.
There is a real thing as dawn phenomenon and it will falsely give readings that are high.
I tried metformin to drive my glucoses down some, but there must be something wrong with me because I always feel like crap on that drug, and always wind up trashing it.
Hi Paul – I know Blagosklonny has written about rapamycin and fasting glucose as a beneficial, physiological insulin resistance, and I agree with him (and you of course). Yet there are many papers in the literature stating that rapamycin “causes” diabetes, which I think is the result of scientists generally not knowing about how insulin resistance works and why CR and fasting can cause it in the liver. As for metformin, I understand that GI upset is a common problem; is that your experience? If rapamycin produces beneficial liver insulin resistance, which appears to be the case, then perhaps metformin isn’t necessary to lower blood sugar.
Hi PD
As usual you know more about it than most doctors. No, my issue isn’t GI , every AMPK activator from metformin, berberine, gynostemma, to the life extension product give me fatigue after vigorous exercise . As you know , exercise itself upregulates AMPK and I guess it’s just too much to add in an exogenous activator. Maybe most diabetics aren’t exercising vigorously enough to feel the detrimental effects. I don’t really know. But I’m going to take your advice about the glucose levels.
Paul, that’s interesting. I’m currently varying berberine, either once every other day or once a day, and I can’t say I can tell any difference. However, I think I’ll try omitting it for awhile to see if it makes a difference. I don’t have the world’s greatest exercise recovery at my age, maybe that’s affecting it.
Which brings me to another point: if exercise and fasting activate AMPK and Nrf2 (which they do), how much do other activators help? In the case of metformin, which lowers mortality rates in humans, the humans in question who take it are diabetics and/or overweight. Would metformin extend lifespan in people who are in good shape and eat well? I think there’s a blindness in a lot of anti-aging science that doesn’t see the importance of lifestyle, even when it comes to lab animals, who are fed crappy food and kept in cages. I wrote about this in one of my articles. Lab rats steadily gain weight through the whole colurse of their lifespan, which seems unlikely to happen in the wild; hence perhaps lab conditions themselves are obesogenic and pro-aging. Metformin may prolong their lives. But would it do so among wild rats? A question that hasn’t been answered.
I think that you are totally correct that there’s two totally different situations here. One is the unfit, overweight diabetic who shuns exercise like the plague and has a high insulin, IGF-1,and all of the markers of metabolic syndrome. To them metformin is a lifesaver and actually prolongs their life.
But to the healthy and fit I think that it’s a different story. We rely on the liver’s gluconeogenesis process and this can be inhibited by AMPK activators. This is especially bad for sprinters and weight lifters. I have many times experimented on myself with one week on and one off with these agents, and I always feel better off of them.
I’m starting to think that total FBS is overrated the way that total cholesterol is and needs to be seen as part of the total picture and not in isolation.
Anecdotally on the low carb/paleo forums many report a slow increase in FBG over the years. Seems to be a reaction to the diet and not the dawn phenomena. Maybe it could be staved off with some more, or at intervals, carbs.
You have a great site! Lots of real, like, you know, SCIENCE!
Thanks, pzo. I cite the scientific literature for every point I make (or try to), and if I speculate, I clearly note that I’m doing so, or that it’s my opinion, etc. There’s just too much pseudoscience or scientific illiteracy floating around on health and fitness sites. Also, since my approach is quite different from the mainstream (weightlifting, low carb whole foods and/or keto, keeping iron low, etc) citing the literature helps me avoid looking like a crazy person.
Dennis, did you see this piece on a mutation in some Amish that seems to protect against diabetes?
The mutation causes lower levels of the PAI-1protein, and is associated with 28% lower levels of insulin in carriers than non-carriers. The reason I thought to mention it here is that while carriers with one copy of the mutation have a lower risk of diabetes, carriers with two copies get a bleeding disorder. Is the mutation causing carriers to have a lower iron load?
NB it looks like n = 177 for this study.
Thanks, I had not seen that. Interestingly, they had lower levels of plasminogen activator inhibitor (PAI-1), which promotes blood clotting. Hypercoagulability is a major problem in aging, leading to strokes, heart attacks, and cancer. The Amish with the mutation lived an astounding 10 years longer than those without the mutation. HUGE effect. The article discusses protection against diabetes, and the fact that homozygous carriers have a bleeding disorder, so there’s a possibility that iron is involved. Iron and consequent bacterial growth are likely highly involved in hypercoagulation.
Why do you keep removing my email address from subscribers list? Apparently eduemail is good? If it’s removed again I won’t return to this site
Sn, I have never removed your or anyone else’s email from my subscriber list. I rarely email my articles anymore which is probably why you don’t get them. I suggest you sign up on the comment form for WordPress updates, which go out automatically.
BTW, pretty outrageous to take umbrage at an imagined and non-existent slight when I provide everything on this site free of charge.
Have you checked your A1c level? I was told, by my practitioner, that even though my glucose was 97, that my A1c was 4.9 which removed all suspicion of prediabetes in her mind. She didn’t mention the dawn phenomenon but she said A1c was a much more accurate test.
Hi Scott, I’ve never had an A1c, but I believe your practitioner is right.